On August 18, 2015, the FDA approved Addyi (flibanserin), a drug to treat “acquired, generalized hypoactive sexual desire disorder (HSDD) in premenopausal women.” The FDA defines HSDD as “low sexual desire that causes marked distress or interpersonal difficulty and is not due to a co-existing medical or psychiatric condition, problems within the relationship, or the effects of medication or other drug substance.”
That’s an interesting parsing of words since some of those parameters may not be mutually exclusive. (And what about situations where men are no longer desirable because of issues related to THEM?)

Much has been written in the last week in light of the FDA approval for Addyi. There are so many things that have me shaking my head. My first thought? Equality is nowhere near the same thing as equity.
It’s not “female Viagra.”
If you were following the news over the last two weeks, you likely heard Addyi referred to as the “female Viagra,” yet the FDA announcement said, “Prior to Addyi’s approval, there were no FDA-approved treatments for sexual desire disorders in men or women.”
The drug is nothing like Viagra: it increases desire by altering women’s brain chemistry, whereas Viagra increases blood flow to the penis. Tara Haelle at Forbes nails it well (ahem) in her post: Female Viagra? Everything You Wanted to Know About Sex Drug Flibanserin But Were Afraid To Ask.
Over at FierceBiotech, John Carroll says the FDA blundered badly on the Addyi approval and says that manufacturer Sprout Pharmaceuticals is benefitting from the viral hype around the “female Viagra” terminology. The FDA never invoked the “female Viagra” wording, but there was a concerted effort by the manufacturer to market a drug for female sexual disorder and others have written about how this drug approval rode in on the tide of the efforts by Even the Score, a collaborative initiative by “26 Organizations who believe that it’s time to level the playing field when it comes to the treatment of women’s sexual dysfunction.”
The manufacturer has no idea how Addyi works.
Originally an antidepressant that failed to win approval two times over, the drug works in the brain by altering neurotransmitters. However, the prescribing information states that the mechanism of action is unknown. This is disappointing, however not unusual given that there are a lot of drugs that have unknown mechanisms of action, e.g. anesthesia.

Mechanism of Action: Addyi Prescribing Information. Source: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/022526lbl.pdf
A lot of fuss and risk for a little more frisk.
Addyi has a lot of drawbacks. The drug:
- must be taken daily and takes several weeks to take effect
- only works for about 1 in 10 women who take it (9-14%)
- provides women who respond with less than one more sexually satisfying events per month (0.5-0.7)
- is recommended to be taken at bedtime because it has a sedating effect on the central nervous system: “Patients should not drive or engage in other activities requiring full alertness until at least 6 hours after taking Addyi and until they know how Addyi affects them.” (But what about women who would like to enjoy sex fully alert? Should they set an alarm for 4 am? Or plan for afternoon escapades?)
- carries significant risks if consumed with alcohol. There is a black box warning on the label about low blood pressure and fainting when patients taking Addyi also consume alcohol (as well as some other medications). Read Dr. Jen Gunter’s excellent post on this risk: Want to take flibanserin (Addyi) for low sex drive? You can’t drink alcohol. Ever.
Dr. Gunter blogged about two other drawbacks. First, the fact that the chance women will feel sick taking the drug is greater than the chance it will help: Flibanserin (Addyi) is more likely to make you sick than horny. What do you think about that?
Second, she flagged for me that in the safety studies about alcohol, which were conducted among a small group of 25 people, 23 of the participants were MEN! Given that women are more susceptible to the toxic effects of alcohol than men, this is alarming. (This reminds me of the ridiculous refusal by some study authors last year to retract a paper from JAMA even after they admitted to including WOMEN in their study about the effects of testosterone replacement therapy and cardiovascular health in MEN…)
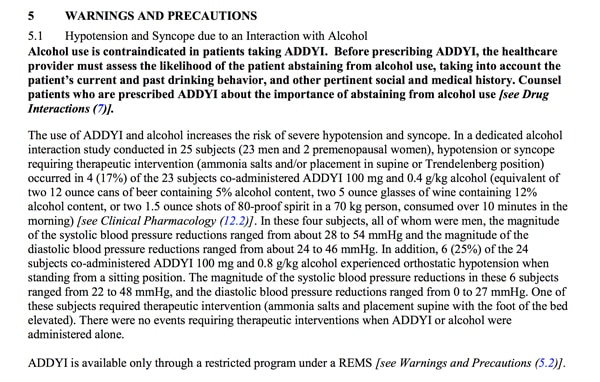
Warnings and Precautions: Addyi Prescribing Information. Source: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/022526lbl.pdf
Is HSDD an invented disorder?
This paper in the Journal of Medical Ethics published online June 29, 2015, says that there is no established norm for sexual activity, feelings or desire, and concludes, therefore, that female hypoactive sexual disorder does not exist:
Condition branding is a marketing technique in which companies develop conditions concurrently with developing drugs; examples include gastro-oesophageal reflux disease, premenstrual dysphoric disorder, social anxiety disorder, erectile dysfunction and hypoactive sexual desire disorder. Although it is illegal for pharmaceutical companies to market drugs prior to regulatory approval, there are no restrictions on marketing diseases, and industry seeks to establish a disease state in the minds of clinicians years before an expected drug launch. Continuing medical education (CME) courses are an important part of promotion prior to drug approval and have become a key marketing tool for increasing clinician receptivity to new products. We systematically identified 14 free, internet-based, industry-funded, accredited CME modules on hypoactive sexual desire disorder in women which came out before a new drug, flibanserin, was being considered for regulatory approval in the USA. Common themes in these modules included the following: (1) Hypoactive sexual desire disorder is common, underdiagnosed and can have a profound effect on quality of life. (2) Women may not be aware that they are sick or distressed. (3) Simple questionnaires can assist clinicians in diagnosing the disorder. (4) It is problematic that there are medicines available to treat sexual problems for men but not women. In fact, there is no scientifically established norm for sexual activity, feelings or desire, and there is no evidence that hypoactive sexual desire disorder is a medical condition. Hypoactive sexual desire disorder is a typical example of a condition that was sponsored by industry to prepare the market for a specific treatment. (Bold emphasis mine).
Nomenclature follies.
Why is there no mention in Addyi’s prescribing information for the condition needing to be present for at least six months before diagnosing a woman with the disorder? The FDA’s summary of patient impact and scientific meetings held last October stated that HSDD falls under female sexual interest/arousal disorder (FSIAD) in the Fifth Edition of the Diagnostic and Statistical Manual (DSM-5), and mentioned the fact that the disorder needs to be present for 6 months before diagnosis. Yet there is no mention of this in the prescribing information that I can see.
The FDA approval was for HSDD, and the FDA meetings held last October discussed female sexual dysfunction (FSD) as an umbrella term: “The term FSD covers a heterogeneous collection of conditions that have previously been classified into four different disorders: hypoactive sexual desire disorder (HSDD) characterized by a reduced or absent interest in sexual activity, female sexual arousal disorder (FSAD) characterized by an inability to attain or maintain sexual excitement, female orgasmic disorder characterized by the difficulty to attain orgasm despite sufficient arousal, and sexual pain disorder characterized by pain during sexual intercourse.
In May 2013, HSDD and FSAD were combined into a single diagnosis of female sexual interest/arousal disorder (FSIAD) in the Fifth Edition of the Diagnostic and Statistical Manual (DSM-5). For a woman to be diagnosed with FSIAD, her symptoms of reduced sexual interest/arousal must have been present for at least six months, and must be severe enough to be a source of personal distress. FSIAD can be lifelong or acquired, range from mild to severe, and may be generalized or situational. There is currently no precise measure of the prevalence of FSIAD. However, one survey of U.S. women found that 12% of women reported experiencing personally distressing sexual problems. (Bold emphasis mine). Source: (PDF) The Voice of the Patient, A series of reports from the U.S. Food and Drug Administration’s (FDA’s) Patient-Focused Drug Development Initiative, Female Sexual Dysfunction Public Meeting: October 27, 2014 Report Date: June 2015.
What’s next for Addyi?
Health Canada often follows FDA approval, but not always. Interestingly, two days after the FDA approval, Addyi’s manufacturer, Sprout Pharmaceuticals of Raleigh, North Carolina was bought for $1 billion by Canadian company Valeant. The company plans to request Health Canada approval this fall.

